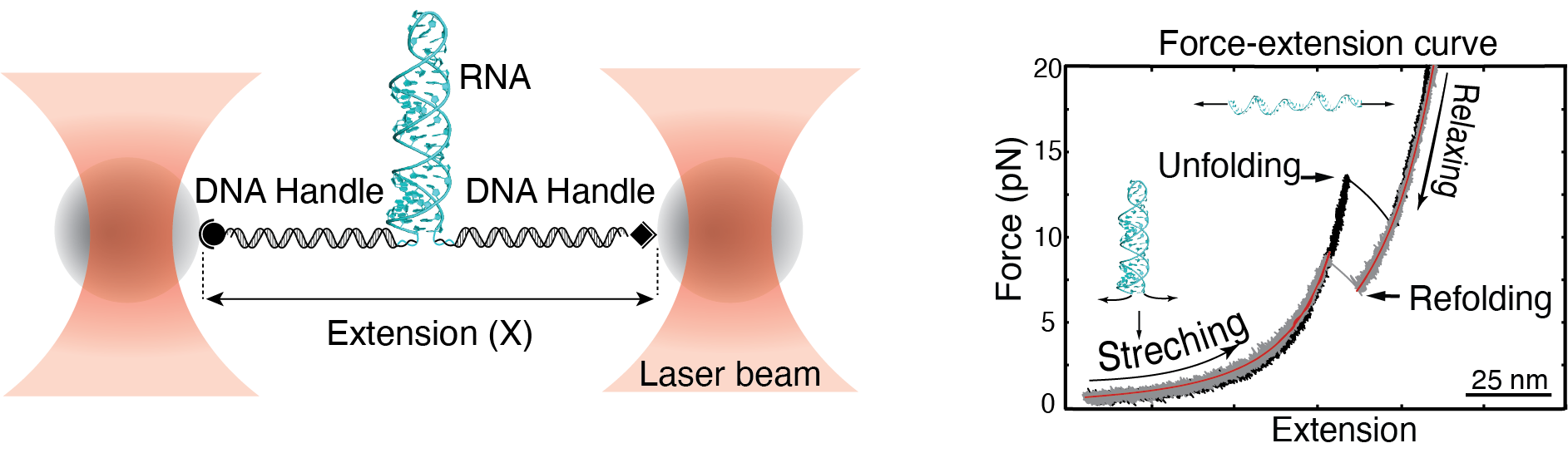
1) RNA Structural Dynamics and Regulation by Single-Molecule Optical Tweezers
We use single-molecule optical tweezers to study how RNA folds, misfolds, and interacts with ions and proteins. Our work focuses on how structural switches in RNA respond to post-transcriptional modifications and how these changes influence regulatory processes such as splicing and translation. By mechanically probing individual RNA molecules, we uncover the physical mechanisms by which RNA structure and dynamics encode molecular regulation at the single-molecule level.

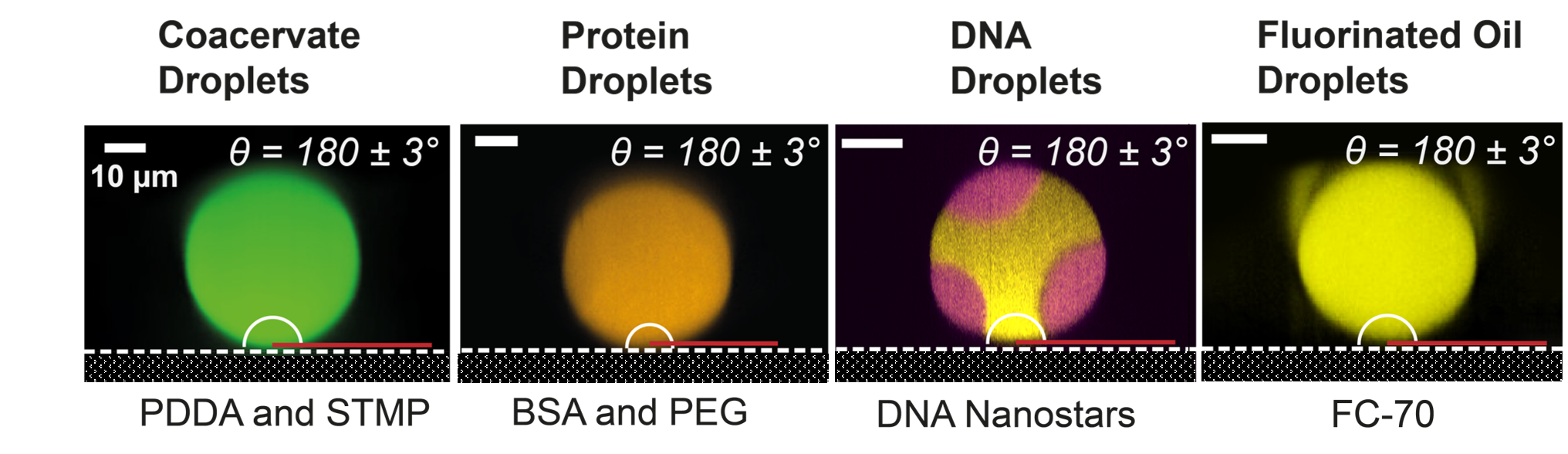
2) Macromolecular Crowding and Biomolecular Phase Separation
We study how the crowded molecular environment of the cell governs the organization and interactions of biomolecules. Using analytical ultracentrifugation, we quantify second virial coefficients as a direct measure of macromolecular crowding and intermolecular forces. These quantitative parameters allow us to predict phase behavior and construct phase diagrams for proteins and polymers under physiologically relevant conditions. By linking crowding, excluded volume, and phase separation within a unified physical framework, we aim to uncover general principles that describe how complex biomolecular mixtures self-organize inside living cells.

3) Shaping and Mechanics of Lipid Membranes
This project investigates how lipid bilayer membranes deform, adhere, and remodel under mechanical forces. Using giant unilamellar vesicles as model systems, we study how membrane bending, surface tension, and adhesion energies govern the formation of flattened, tubular, and stacked morphologies reminiscent of cellular organelles. We are developing a quantitative experimental platform that allows controlled reconstitution of proteins and lipids to directly measure how they generate and respond to membrane shape changes. These insights ultimately provide a basis for understanding how membrane mechanics enable selective molecular sorting and organization in living cells.

4) Phase Behavior and Self-Assembly of Rod-like Proteins
We explore how rod-like proteins organize and phase-separate to form ordered, functional assemblies. Building on discoveries that coiled-coil proteins and DNA origami nanorods can undergo liquid–liquid phase separation, we study how molecular shape and anisotropy give rise to emergent collective behaviors. Such systems provide powerful minimal models for understanding how directional interactions drive assembly in biological and synthetic contexts. By combining biophysical experiments with model protein systems, we aim to uncover the principles that govern the transition from disordered mixtures to highly organized structures such as colloidal membranes—ultrathin, fluid layers of aligned filaments that resemble biological membranes in form and function. These studies reveal how geometry and interaction symmetry shape the mesoscale organization of matter, offering new routes toward designing programmable, life-like materials.

5) Light-Controlled RNA Granules in Synaptic Plasticity
Neurons rely on tightly regulated, localized protein synthesis to strengthen or weaken specific synapses during learning and memory. This process is coordinated by dynamic RNA granules—biomolecular condensates that assemble and dissolve to control when and where translation occurs. In our lab, we study how these RNA granules form, how chemical modifications such as m⁶A alter their behavior, and how their dynamics shape synaptic signaling and plasticity. By combining tools from molecular biophysics, neurobiology, and photonics, we aim to reveal how light or other precise stimuli can modulate RNA-based regulation of protein synthesis in neurons, offering new insight into the molecular basis of memory and its dysfunction in disease.

6) Mechanisms of Super-Omniphobicity
Super-omniphobic surfaces repel nearly all liquids—water, oils, and complex biological fluids—by preventing spreading and pinning even under stress. We study the physical and chemical principles that enable such extreme repellence, focusing on how interfacial structure and elasticity govern wetting transitions. By integrating polymer physics, surface chemistry, and fluid mechanics, we explore how hydrogel-based interfaces sustain non-wetting states beyond classical models. Our goal is to uncover the mechanisms of hydrogel-driven super-repellence and translate these insights into durable, anti-fouling, and self-cleaning coatings for use in complex environments.